usp class vi elastomers
Colorites Cellene line of thermoplastic elastomer compounds is an alternative to PVC phthalate-based plasticized compounds and various rubber materials in medical devices medical packaging and other regulated markets. Ia USP Class VI andor ISO 109933 will be required.

Parker Medical Fda O Rings Sealing Devices
A workshop Modernization of USP Packaging Standards for Glass and Elastomeric Components will take place June 1920 2017 at the USP Meetings Center in Rockville Maryland to discuss the proposals for three new chapters including this one Elastomeric Closure Functionality in Injectable Pharmaceutical PackagingDelivery Systems 382 and.

. USP Class VI elastomers. Master Bond systems are very versatile and can be used for both disposable and. These medical silicones come in thicknesses between 005 and 250 and in the following durometers.
The USP publishes bio compatibility protocols for the plastics and polymers used in medical devices or surgical equipment that may come in. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal. Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements.
Specially formulated for long term sealing. Additionally 22 Material Certifications certifying the composition of all materials in the wetted path and International Calibration. The SSP2390 family of products isnt new but the marketplace for these materials continues to evolve.
Most applications are fairly benign to elastomers. Darcoid and Parker offer a wide range of USP Class VI and FDA. 7 USP Class VI materials.
Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant. Valley Seal offers a wide range of USP Class VI and FDA elastomeric materials for the Healthcare Industry. As a result any rubber compounds certified under USP Class VI are proven to have high levels of biocompatibility meaning they are ideally placed to use in medical and pharmaceutical situations.
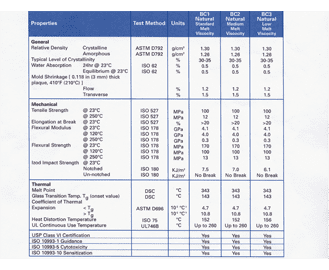
10 20 30 40 50 60 70 and 80. FDA and USP Class VI O-ring materials for life sciences Parker Compound Polymer Hardness Color Temperature Range F Service EJ280-70 EPDM 70 Black -70 to 250 FDA USP VI Animal-free E3609-70 EPDM 70 Black -70 to 250 FDA USP VI FF156-75 FFKM 75 Black 5 to 525 Broad chemical resistance USP Class VI. JBC can also source materials in custom colors but the pigments.
Any company wishing to produce USP Class O-rings for any kind of food. Pharmacopoeia Class VI judges the suitability of plastic material intended for medical device manufacturers. Pharmacopeial Convention USP is a non-profit organization with a purpose of creating standards for medications food ingredients dietary supplements and healthcare technologies.
Find Usp Approved Elastomers related suppliers manufacturers products and specifications on GlobalSpec - a trusted source of Usp Approved Elastomers information. June 15 2017 By Lee Goldberg. Both products are optically clear and they meet the USP class VI specifications.
USP Class VI and FDA u001c White Listu001d Silicone and Organic Elastomer Compounds for Healthcare Products. Food and Drug Administration FDA. USP Class VI Approved Medical Grade Plastic Materials - USP - US.
FDA and USP Class VI O-Rings. Class VI elastomers are highly sought after by those in the medical and pharmaceutical industries. There are two important organisations that play a key role in the regulation of elastomers and O-rings these being the United States Pharmacopeia USP and the US Food and Drug Administration FDA as well as meeting European regulation EU1935.
The Premium Package includes all elastomers in the wetted path of the SLA Series Biotech including O-rings and Valve Seats are USP Class VI and ADI Free and include a full package of certificates. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for Healthcare Products. These materials may be blended if desired to achieve intermediate hardnesses.
What are Class VI elastomers. To keep up with the changes medical device designers and manufacturers need elastomers they can trust. Sanitary diaphragm valves have USP Class VI diaphragms.
These materials can be supplied as precision extruded and cut seals used as static face or static radial seals long length. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. Most applications are fairly benign to elastomers.
Please contact the division for assistance in selecting materials in these situations. USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. USP Class VI refers to a set of biocompatibility testing requirements from the US.
It resists many chemicals including water oils fuels and. Category Featured Company Information. Sanitary pumps require Class VI O-rings and seal material.
Who uses USP Class VI elastomers. Two component EP21AOLV-2Med passes USP Class VI biocompatibility testing and ISO 10993-5 cytotoxicity requirements. For more information on UPS Class VI seals or to place your order contact Eastern Seals by phone 44 0 1670 840529 or email saleseasternsealscouk.
Sil 714001 USP class VI Silicone 1 70 Yes transl. Isolast Plus J9515 J9516 - Superior sealing with FDA USP class VI and SP 3-A compliance. Tire Rubber Elastomer Products 3011-TR Touchscreens Graphic Overlays Membrane Switches 3575-TS Trade Show Display Events 2542-TD Trading Companies.
All these special grade products have passed this rigorous test. The FDA USP Class VI silicones that JBC sources are translucent in color and supplied as compression-molded sheet stock or continuous rolls. Pharmacopeia USP a non-profit organization whose standards inform decision-making at the US.
In addition UV10TKMed also meets the ISO 10993-5 specifications. However some applica-tions such as implantable devices are extremely complicated. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity.
Liveo Class VI Elastomers C6- eries are a series of one-part uncatalyzed silicone elastomer raw materials. Thermoplastic Elastomers for Medical Devices Meet USP Class VI and ISO 10993 Standards. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and.
The cross-linked molecular chains enable them to combine the resilience and sealing force of an elastomer with the chemical inertness and thermal stability of PTFE. Specialty Silicone Products SSP makes USP Class VI silicones for medical applications. Sil 714002 USP class VI Silicone 1 70 Yes transl.
UV10Med offers very low viscosity in comparison to UV10TKMed which offers moderate yet flowable viscosity. The resulting elastomers range in hardness from soft to firm nominally 35 to 65 shore A durometer.

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California

Fda And Usp Class Vi O Rings Guide 2020 Nes

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Pharmaceutical And Cosmetics Production

Usp Class Vi Seals Compliant To Food Grade Standards Barnwell

Valley Seal Introducing Usp Fda Specialty Elastomers
![]()
Usp Vi Silicones Fda Approved Pronat

Meaning Of Usp Class Vi Standard United Kingdom

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc
Meaning Of Usp Class Vi Standard United Kingdom

Reliable High Performance Parylene Dimers Vsi Parylene
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket


